 |
- Day 1:
- Digest DNA
- Run an agarose gel overnight
- Day 2:
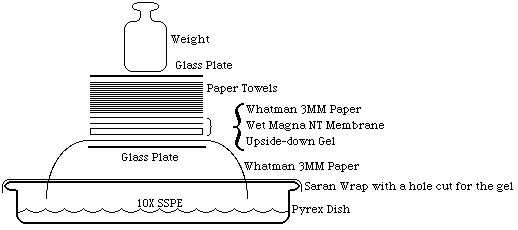
- Blot gel
- Label the probe
- Day 3:
- Dry membrane (can be left at this point)
- Purify probe
- Pre-hyb
- Hyb overnight
- Day 4:
- Post hyb
- Expose film overnight
- Day 5:
- Develop film
- Strip membrane
 |
5 µl Oligo Labeling Buffer (OLB) 1 µl 10 mg/ml BSA (nuclease-free) 0.5 µl dA/dT/dG mix (1mM each) 2 units Klenow fragment of DNA Polymerase I
2.5 µl alpha-(32P)-dCTP (25 µCi) (3000 Ci/mmole)
1 µl 0.25M EDTA 1 µl 10% SDS
50 ml NH4 Formate (0.3M pH=7.8) 50 ml NH4 Carbonate (0.25M) 25 ml 95% EtOH
Reagent [Stock] Volume [Final] Formamide - 12.5 ml 50% SSPE 20X 6.25 ml 5X Denhardt's 50X 5.0 ml 10X SDS 20% 1.25 ml 1% Salmon Sperm DNA 10 mg/ml 0.75 ml 300 µg/ml
Reagent [Stock] Volume [Final] SDS 20% 25 ml 1% SSPE 20X 50 ml 2X H2O - 425 ml -
Reagent [Stock] Volume [Final] SDS 20% 15 ml 0.2% SSPE 20X 15 ml 0.2X H2O - 1470 ml -
Reagent [Stock] Amount [Final] SDS 20% 2.5 ml 0.1% NaOH - 4 g 0.2N H2O - to 500 ml -