Southern Blot
Based on Maniatis
Schedule:
- Day 1:
- Digest DNA
- Run an agarose gel overnight
- Day 2:
- Blot gel
- Label the probe
- Day 3:
- Dry membrane (can be left at this point)
- Purify probe
- Pre-hyb
- Hyb overnight
- Day 4:
- Post hyb
- Expose film overnight
- Day 5:
- Develop film
- Strip membrane
Blotting the Gel:
Use powder-free gloves.
- Stain the gel in 10 mg/l Ethidium bromide. Take a picture to see how well the enzymes cut, include a ruler.
- You can, if you so desire, cut the wells off of the gel. Trim one corner of the gel as a reference.
- Acid wash the gel for 8 minutes in 10.4 ml conc HCl + 489.6 ml dH2O.
- Rinse once with dH2O.
- Denature 2 times (15 minutes each) in 58.6 g NaCl + 20 g NaOH / liter
- Rinse twice with dH2O.
- Neutralize 2 times (15 minutes each) in 30.3 g Tris + 43.9 g NaCl + 16.75 ml HCl / liter.
- Rinse twice with dH2O.
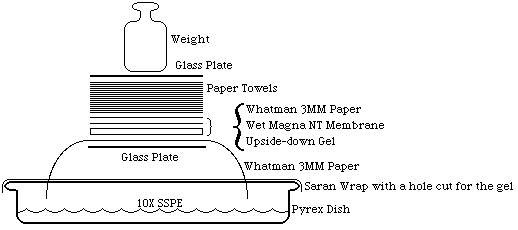
- Blot overnight onto Magna NT with 10X SSPE. Write name on membrane with a ballpoint pen. Wet membrane in 10X SSPE and set up as shown:
Labeling the Probe
- Bring 50ng of the DNA to be labeled to a volume of 17 µl with H2O.
- Boil for about 3 minutes to denature the DNA.
- Chill on ice.
- Add the following to the DNA sample:
5 µl Oligo Labeling Buffer (OLB) 1 µl 10 mg/ml BSA (nuclease-free) 0.5 µl dA/dT/dG mix (1mM each) 2 units Klenow fragment of DNA Polymerase I
- In the Hot Lab, add
2.5 µl alpha-(32P)-dCTP (25 µCi) (3000 Ci/mmole)
- Incubate at 37°C for at least 3 hours or at room temperature overnight.
Drying the Membrane
- Stain the gel and photograph. Did all the DNA transfer?
- Wash the membrane in 5X SSPE.
- Put the membrane on a piece of Whatman paper wetted in 10X SSPE, which is on a piece of dry Whatman paper. Crosslink @ 1200.
- Let the membrane sit on the bench for about 5 minutes.
- Bake for 2 hours at 80°C under vacuum.
- Store at room temperature.
Purifying the Probe
- To stop the reaction add:
1 µl 0.25M EDTA 1 µl 10% SDS
- Spot 1 µl of the stopped reaction onto each of two small pieces of DE81 filter.
- Wash one of the pieces of DE81, using the vacuum flask filter thing, with:
50 ml NH4 Formate (0.3M pH=7.8) 50 ml NH4 Carbonate (0.25M) 25 ml 95% EtOH
- Measure the activity of both pieces of filter in the scintillation counter to determine the percent incorporation (70% is good).
- Plug the bottom of a 1 ml disposable syringe with a little bit of glass wool and put the syringe in 15 ml conical tube.
- Fill the syringe with Sephadex G-50 in STE Buffer (TE + 100 mM NaCl + 0.02% NaN3).
- Spin the tube/syringe in the clinical centrifuge at top speed for 2 minutes. Add more Sephadex and spin again. Repeat until the packed Sephadex volume is approximately 0.9 ml. [image missing]
- Cut the lid off of a 1.5 ml Epindorf tube. Place the decapped tube in another 15 ml conical tube. Put the syringe in the new tube so that the tip of the syringe is in the Epindorf tube.
- Add enough STE to the stopped probe reaction to bring the volume to 100µl.
- Transfer the 100µl probe reaction to the top of the Sephadex-packed syringe. Cover the top of the spin column with Parafilm.
- Spin the column in the clinical centrifuge for 2 minutes at top speed.
- Wash the reaction tube with 100µl STE. Add it to the column and spin for another 2 minutes.
- Remove the labeled probe from the Epindorf tube with a Pasteur pipette and dispose of the waste in the appropriate containers.
- Spot 1µl of the probe solution a a piece of DE81 filter. Put the filter in a scintillation vial and add 2 squirts of scintillation fluid. Measure the activity of the probe using the scintillation counter. A final concentration of 106 to 2 x 106 cpm per ml of hybridization solution is optimal.
- Before adding the labeled probe to the hybridization solution, boil it for 3 minutes and chill on ice.
Prehybridization and Hybridization
- Make 25 ml of prehyb solution and add it to a heat sealable bag with the membrane. Seal the bag.
Reagent [Stock] Volume [Final] Formamide – 12.5 ml 50% SSPE 20X 6.25 ml 5X Denhardt’s 50X 5.0 ml 10X SDS 20% 1.25 ml 1% Salmon Sperm DNA 10 mg/ml 0.75 ml 300 µg/ml
- Seal the bag in a box of water and shake at 42°C in the dry shaker for 4 hours.
- Cut the bag at one corner and drain out the prehyb solution.
- Make another 25 ml of prehyb solution and add it to the bag through the cut corner. Add the heat denatured probe to the bag (prehyb solution + probe = hyb solution). Seal the bag.
- Incubate with shaking at 42°C overnight.
Posthybridization
- Cut the bag and drain the hybridization solution into a 50 ml plastic tube. Label the tube and store at -20°C. It can be reused several times.
- Wash the membrane once with 500 ml 2X SSPE, 1% SDS for 25 minutes at room temperature.
Reagent [Stock] Volume [Final] SDS 20% 25 ml 1% SSPE 20X 50 ml 2X H2O – 425 ml –
- Wash membrane 3 times with pre-heated 0.2X SSPE, 0.2% SDS for 20 minutes at 65°C to 68°C.
Reagent [Stock] Volume [Final] SDS 20% 15 ml 0.2% SSPE 20X 15 ml 0.2X H2O – 1470 ml –
- Expose X-Ray film.
Stripping the Membrane
- Rinse the membrane in sterile, distilled H2O.
- Incubate the membrane in 0.2N NaOH, 0.1% SDS at 42°C with shaking for 30 minutes.
Reagent [Stock] Amount [Final] SDS 20% 2.5 ml 0.1% NaOH – 4 g 0.2N H2O – to 500 ml –
- Wash in 2X SSPE
- Wrap in Saran Wrap, squeezing out extra moisture with a pipette and store at -20°C. To re-probe, begin at the pre-hybridization stage.
This page is, as always, Copyright (c) 1996 by Craig D. Amundsen. All rights reserved.